
The battery is programmed by the health team to send a few seconds of electrical energy to the vagus nerve every few minutes.
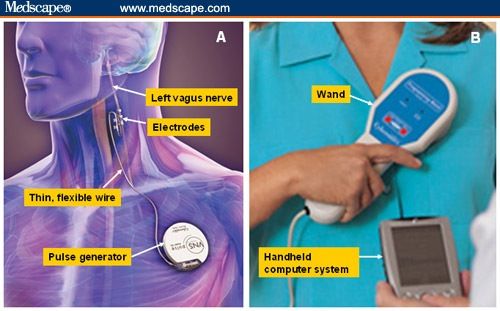
#Vagus nerve stimulator skin
Thin wires (electrodes) are threaded under the skin and wound around the vagus nerve in the neck. The energy is delivered by a flat, round battery, about the size of a silver dollar, which is surgically implanted in the chest wall. The therapy is designed to prevent seizures by sending regular small pulses of electrical energy to the brain via the vagus nerve, a large nerve in the neck. It is currently approved for use in adults and children over the age of 12 who have partial seizures that resist control by other methods. “The VNS device is a nice option to have, but cost can be a limiting factor.Vagus Nerve Stimulation therapy is another form of treatment that may be tried when medications fail to stop seizures. “That tends to be the biggest barrier for care,” she says. Hamilton notes is “pretty steep.” She notes that there are instances where insurance will cover the cost of the device, but this does not extend to all cases. The cost per month out-of-pocket is about $700, which Dr. Patients essentially rent a VNS device, she says, and every month it needs to be reactivated. Hamilton says she thinks the biggest limitation for most patients is cost. “However, in a lot of populations, it’s very safe to use.” Does this treatment have any barriers to access?ĭr. The device does, however, affect heart rhythm, making it potentially dangerous for patients with heart conditions. It is safe for use in many different patient groups, and is currently being studied for patients who are pregnant-a population with great need due to very limited options for migraine or cluster headache treatment during pregnancy. Hamilton says there is already research demonstrating the benefit of the VNS device. Who is a good candidate for this treatment?ĭespite the lack of long-term data, Dr. The device is also not associated with any significant side effects. Hamilton indicated that the number is so high that it’s unlikely any patient will reach that threshold, making the device safe to use multiple times a day.įurthermore, the VNS device is non-invasive, greatly reducing the potential for complications such as surgery-related infection. While there is an upper limit of how many times a patient can use the VNS device, Dr. This neuromodulation device is ideal for patients who have frequent migraine attacks who don’t wish to take too many as-needed medications-like abortives-that can result in medication overuse headache. What are the benefits of VNS compared to other treatments? This is an exciting development for the cluster headache community because there are currently no other preventive treatments that have been FDA-approved or cleared for cluster headache. The device has been shown to significantly lower the number of cluster attacks per week, compared with patients using only their regular medications.

In January 2019, VNS was approved for the prevention of cluster headache.
#Vagus nerve stimulator trial
The trial evidence showed that VNS decreased the pain level of a migraine at 30 minutes and 60 minutes after using the device.

In the spring of 2018, VNS was FDA-cleared as an acute or abortive treatment for migraine. Katherine Hamilton, a neurologist and assistant professor of clinical neurology at the University of Pennsylvania, weighed in on how VNS can help patients manage migraine and cluster headache.
In November 2018, the FDA cleared the VNS device for treatment of cluster headache. Recent clinical trials have been evaluating vagus nerve stimulation (VNS) for the treatment of various headache conditions, including migraine and cluster headache. Vagus Nerve Stimulation for Migraine and Cluster Headache This non-invasive, FDA-cleared treatment option decreases the likelihood of headaches


 0 kommentar(er)
0 kommentar(er)
